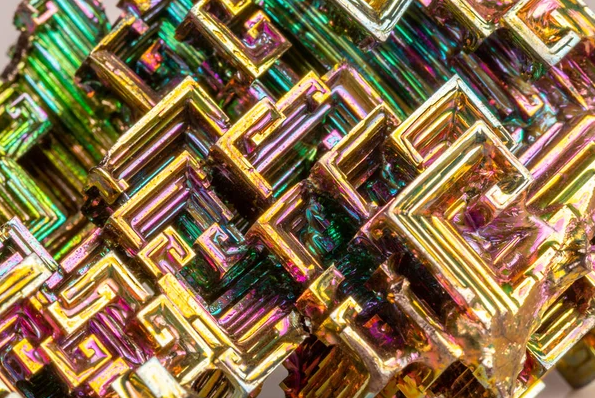


Bismuth is a fascinating element with properties that set it apart from others in the periodic table. Its low thermal conductivity and high electrical resistance make it an excellent material for certain applications in electronics and manufacturing. What truly captures the imagination, however, are its iridescent crystals. These crystals form due to the unique arrangement of atoms in its crystalline structure, resulting in stunning displays of colors reminiscent of a rainbow. Bismuth's distinctive appearance not only makes it a favorite among collectors but also plays a crucial role in scientific research, where its properties are studied to unlock new possibilities in various fields.
Bismuth has a rich and storied history that spans millennia. It was known to ancient civilizations and used in various forms, including cosmetics and medicines. However, its true nature as a distinct element wasn't discovered until the 18th century. Since then, bismuth has found its way into a multitude of industrial processes and modern technologies. Its applications range from alloys and pigments to pharmaceuticals and electronics. The journey of bismuth from ancient times to the present day is a testament to its versatility and enduring significance in human civilization.
Bismuth crystals are renowned for their captivating beauty and vibrant colors. These iridescent hues result from the unique structure of bismuth's crystals, which reflect and refract light in intricate patterns. Growing bismuth crystals is both an art and a science, requiring careful control of temperature and other conditions. The resulting crystals vary in size and shape, each possessing its own mesmerizing allure. Beyond their aesthetic appeal, bismuth crystals are also valued for their role in scientific research, where they are used in studies ranging from crystallography to materials science. Whether admired for their beauty or studied for their properties, bismuth crystals continue to captivate minds around the world.
In thermoelectric devices, bismuth-based materials convert waste heat into electricity.
The thermoelectric efficiency, represented by ZT, is crucial.
For a hypothetical material with σ = 1000 S/m, S = 100 μV/K, T = 300 K, and κ = 1 W/mK, ZT = 0.003.
Higher ZT values signify more efficient materials, showcasing bismuth's role in sustainable energy solutions.
Bismuth has a long history of use in medicine, thanks to its unique properties and minimal toxicity. One of its primary applications is in treating gastrointestinal disorders such as indigestion and ulcers. Bismuth compounds act as protective agents, coating the stomach lining and preventing further damage from acid. Additionally, bismuth subsalicylate, a common ingredient in over-the-counter medications, is used to alleviate symptoms of diarrhea and upset stomach. Beyond its gastrointestinal benefits, bismuth is also being explored for its potential antimicrobial properties, with researchers investigating its efficacy against various pathogens. In the realm of medicine, bismuth continues to be a valuable tool in promoting health and well-being.
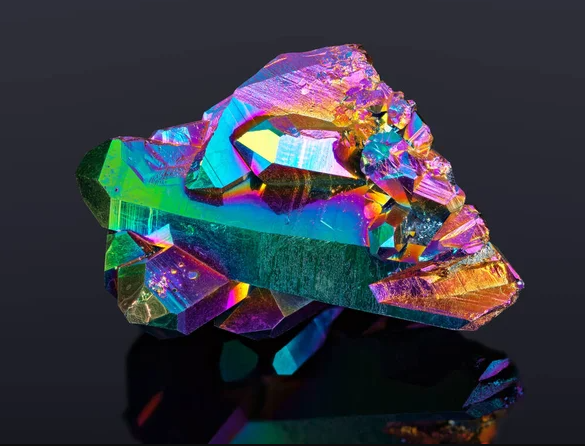
In the realm of electronics, bismuth plays a crucial role in enabling technological advancements. Its unique properties, such as high electrical resistance and low thermal conductivity, make it an ideal material for certain applications. Bismuth is commonly used in semiconductors, where it helps regulate the flow of electrical currents with precision. Additionally, bismuth-based alloys are utilized in thermoelectric devices, where they convert waste heat into electricity, paving the way for more sustainable energy solutions. As the demand for smaller, more efficient electronic devices grows, so too does the importance of bismuth in powering the future of technology.
Bismuth alloys are prized for their ability to enhance the properties of other materials. By combining bismuth with other metals, engineers can create alloys with specific characteristics tailored to particular applications. For example, bismuth-tin alloys are commonly used in soldering due to their low melting points and excellent wetting properties. Bismuth's low toxicity also makes it an attractive choice for safety devices such as fire sprinklers and ammunition. Furthermore, bismuth alloys exhibit superior machinability, allowing for precise shaping and forming in manufacturing processes. Whether improving conductivity, enhancing safety, or facilitating production, bismuth alloys continue to play a vital role in strengthening materials across industries.
Bismuth boasts a favorable environmental profile compared to other heavy metals, thanks to its low toxicity and minimal persistence in the environment. Its use in various applications, such as soldering and ammunition, helps reduce reliance on more harmful substances like lead. However, like any mining and manufacturing process, the extraction and processing of bismuth can have environmental implications if not managed responsibly. Efforts to mitigate these impacts include implementing sustainable mining practices, minimizing waste generation, and ensuring proper disposal of byproducts. By balancing the benefits of bismuth with its potential risks, we can harness its valuable properties while safeguarding the environment for future generations.
At the nanoscale, bismuth nanoparticles exhibit unique properties that hold promise for a wide range of applications. Their small size and high surface area-to-volume ratio make them ideal candidates for use in medicine, catalysis, and imaging. In drug delivery systems, bismuth nanoparticles offer targeted delivery of therapeutic agents, minimizing side effects and improving treatment outcomes. Additionally, their tunable reactivity makes them valuable catalysts for chemical reactions in industrial processes and environmental remediation. In medical imaging, bismuth nanoparticles serve as contrast agents, providing high-resolution images for diagnostic purposes. As researchers continue to explore their potential, bismuth nanoparticles represent a world of possibilities for innovation and advancement in various fields.
Bismuth nanoparticles are synthesized using a chemical reduction approach with NaBH4.
The reaction Bi^3+ + 3NaBH4 + 6H2O → Bi + 3NaBO2 + 9H2 yields nanoparticles.
This method demonstrates bismuth's versatility in applications like drug delivery and catalysis, promising advancements in various fields.
The future of bismuth research is ripe with opportunities for innovation and discovery. Emerging trends point towards advanced materials with enhanced properties, such as bismuth-based alloys for aerospace and automotive applications. Additionally, the integration of bismuth into renewable energy technologies holds promise for more efficient solar cells and thermoelectric devices. In the field of quantum computing, bismuth's unique electronic properties are being explored for their potential in qubit technologies. As researchers push the boundaries of what's possible with bismuth, they pave the way for a future filled with groundbreaking technologies and sustainable solutions.
Bismuth is used in various applications, including medicine (such as Pepto-Bismol), cosmetics, alloys (like solder), and electronics.
Yes, bismuth is generally safe for most uses. It has low toxicity and is used in many pharmaceutical and cosmetic products.
Bismuth is primarily formed through the decay of radioactive elements, such as uranium and thorium, in the Earth's crust.
Bismuth crystals are iridescent structures formed when molten bismuth solidifies. They display vibrant colors due to light interference.
Bismuth has low thermal conductivity, high electrical resistance, and is diamagnetic. It also expands as it solidifies, unlike most metals.
No, bismuth is diamagnetic, meaning it is repelled by magnetic fields and does not retain magnetism.
Bismuth is primarily found as a byproduct of lead, copper, tin, and tungsten mining. It's also found in some minerals like bismuthinite.
Bismuth alloys are mixtures of bismuth with other metals. They have low melting points and are used in applications like soldering.
Natural bismuth is not radioactive, but it can become slightly radioactive through artificial processes, such as neutron bombardment.
Bismuth compounds are used to treat gastrointestinal disorders like indigestion and diarrhea. They help soothe the stomach lining and alleviate symptoms.