


The combined gas law, a synthesis of Boyle's, Charles', and Gay-Lussac's laws, expresses the relationship between the pressure, volume, and temperature of a gas. This law is applicable to ideal gases and real gases under certain conditions. It states that when the pressure, volume, and temperature of a gas change, the product of the pressure and volume divided by the temperature remains constant. This principle is fundamental in various scientific fields, from chemistry and physics to engineering and meteorology, guiding researchers in understanding gas behavior and its practical applications.
Gas laws, including the combined gas law, are governed by mathematical formulas that describe the behavior of gases under different conditions. The combined gas law equation, PV=nRT, encapsulates the relationships between pressure (P), volume (V), temperature (T), and the number of moles (n) of a gas. This formula enables scientists and engineers to make accurate predictions and calculations regarding gas properties and behaviors in diverse scenarios, ranging from industrial processes to environmental studies and beyond.
The combined gas law finds practical applications in numerous real-world scenarios across various industries. For instance, in automotive engineering, understanding how changes in pressure, volume, and temperature affect gas behavior is crucial for designing efficient engines and air conditioning systems. Similarly, in chemical manufacturing, precise control of these parameters is essential for optimizing reactions and ensuring product quality. Moreover, meteorologists rely on gas law principles to model atmospheric phenomena such as weather patterns and climate dynamics, enhancing our ability to predict and mitigate natural disasters.
Gas law calculations involve applying mathematical formulas, such as the combined gas law equation, to solve problems related to pressure, volume, temperature, and the quantity of gas. By following a systematic approach, scientists and engineers can step through these calculations with confidence. This process often begins with identifying known quantities and units, applying the appropriate gas law equation, and manipulating variables to solve for the desired unknown. Through practice and familiarity with gas law principles, individuals can streamline these calculations and make informed decisions in academic, industrial, and research settings.
Formula:
Where:
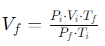
Vf = Final volume of the gas
Pi = Initial pressure of the gas
Vi = Initial volume of the gas
Tf = Final temperature of the gas (in Kelvin)
Pf = Final pressure of the gas
Ti = Initial temperature of the gas (in Kelvin)
Given:
Pᵢ=2 atm
Vᵢ=5 L
Tᵢ=300 K
Pf=3 atm
Tf=350 K
Using the formula:
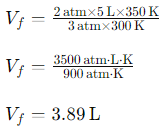
So, the final volume of the gas is 3.89L
Experimental verification of the combined gas law involves conducting controlled experiments to validate the relationships between pressure, volume, and temperature observed in theoretical gas laws. Scientists use specialized equipment, such as gas syringes, pressure sensors, and temperature probes, to collect data under various conditions. By comparing experimental results with theoretical predictions, researchers can assess the accuracy and applicability of gas law principles, contributing to our understanding of gas behavior and informing advancements in fields such as materials science, environmental engineering, and energy technology.

Changes in temperature and pressure significantly influence the behavior of gases, impacting phenomena ranging from weather patterns to chemical reactions. At higher temperatures, gas molecules move faster and exert greater pressure on their surroundings, while lower temperatures result in decreased molecular motion and reduced pressure. Similarly, changes in pressure affect the volume of a gas, following principles outlined in the combined gas law. Understanding these effects is essential for predicting gas behavior in diverse environments and applications, from industrial processes to atmospheric dynamics, and informing strategies for optimization and control.
In industrial processes, the combined gas law plays a critical role in optimizing operations and ensuring product quality. For example, in chemical manufacturing, controlling pressure, volume, and temperature is essential for achieving desired reaction rates and product yields. Similarly, in food processing, maintaining specific gas conditions within packaging helps preserve freshness and extend shelf life. Moreover, in energy production, gas law principles guide the design and operation of power generation systems, optimizing efficiency and minimizing environmental impact. By applying these principles, engineers and manufacturers can enhance productivity, sustainability, and safety across various industries.
In refrigeration systems, gas compression is a crucial process governed by the combined gas law.
As gas is compressed, its pressure and temperature increase, leading to changes in volume and energy transfer.
For example, in a refrigerator, gas is compressed by a compressor, increasing its pressure and temperature.
This compressed gas then flows through a condenser where it releases heat to the surroundings and condenses into a liquid.
The liquid refrigerant then enters an expansion valve, where it undergoes a pressure drop, leading to a decrease in temperature and the absorption of heat from the refrigerator's interior.
Understanding the principles of gas compression and expansion is essential for optimizing the efficiency and performance of refrigeration systems in various industrial and commercial applications.
Gas laws govern atmospheric phenomena such as weather patterns and climate dynamics, shaping our understanding of Earth's climate system. For instance, changes in temperature, pressure, and volume drive atmospheric circulation patterns, influencing wind patterns, precipitation, and temperature gradients. Moreover, variations in greenhouse gas concentrations, governed by gas law principles, contribute to climate change by altering the Earth's energy balance. Understanding these interactions is crucial for predicting future climate scenarios and developing strategies to mitigate the impacts of climate change on ecosystems, economies, and societies worldwide.
Advanced concepts in gas laws extend beyond the combined gas law to include topics such as the ideal gas law and deviations from ideal gas behavior. While the combined gas law provides a useful framework for understanding gas behavior under many conditions, real gases may deviate from ideal behavior due to factors such as molecular interactions and non-uniform conditions. Advanced models, including the van der Waals equation and virial expansion, account for these deviations and provide more accurate representations of gas behavior in complex systems. By exploring these concepts, researchers deepen their understanding of gas properties and refine models for applications ranging from chemical engineering to astrophysics.
The combined gas law is a gas law that combines Boyle's, Charles', and Gay-Lussac's laws into a single equation, relating the pressure, volume, and temperature of a gas.
The formula for the combined gas law is PV=nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.
The gas constant R has units of J / mol⋅K (joules per mole per Kelvin) in the International System of Units (SI).
The combined gas law is applicable to both ideal gases and real gases under conditions of constant temperature and quantity of gas.
Real-world applications of the combined gas law include gas storage, weather prediction, scuba diving, refrigeration, and automotive engineering.
To solve problems using the combined gas law, identify the known variables (pressure, volume, temperature, and moles), rearrange the equation as needed, and solve for the unknown variable.
In chemistry, the combined gas law is significant for understanding the behavior of gases in chemical reactions, gas-phase equilibria, and stoichiometry calculations.
According to the combined gas law, if pressure increases at constant temperature, the volume of the gas decreases proportionally.
The combined gas law is a generalized form of the ideal gas law that accounts for changes in both pressure and temperature, whereas the ideal gas law assumes constant pressure or volume.
Yes, the combined gas law can be used to calculate the density of a gas by rearranging the equation to solve for density (mass per unit volume) when the pressure, volume, and temperature are known.